Bile acids in intestinal immune regulation.
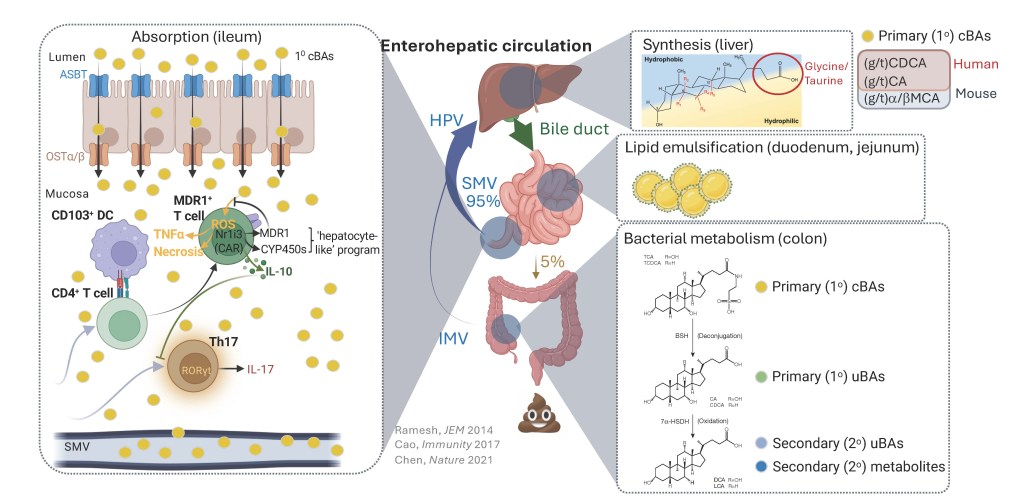
Bile acids (BAs) are core gastrointestinal (GI) metabolites with dual functions in lipid absorption and cell signaling. The past decade has seen a marked resurgence in BA research thanks to seminal studies – including from our lab – that established BAs as direct modulators of intestinal immune responses. Most current efforts in this area are now centered on mapping immunoregulatory functions of isolated BA species. Conversely, our lab’s work is centered on the premise that intestinal immune cells, in both humans and mice, are exposed to large, complex, dynamically regulated and still-poorly defined BA pools. We recently described a novel methods set for quantifying native pools of immune-facing BAs that circulate through intestinal mucosae of mice (Sudo et al., Cell Mol Gastroenterol Hepatol. 2024;18(6):101392). We are now applying these methods and insights to: (i) determine whether, how and why mucosa-associated BA pools might change in settings of GI pathology; and (ii) elucidate how individual species within these BA pools compete for interactions with immune cells and receptors.
Nuclear receptors in intestinal immune regulation
Nuclear receptors (NRs) are a large family of ligand-regulated transcription factors that mediate cellular transcriptional responses to hormones and small molecule metabolites, including bile acids (BAs). We previously discovered the constitutive androstane receptor (CAR/Nr1i3) – a drug- and BA-sensing NR known for mediating phase I/II hepatic metabolism – as required for CD4 T cell homeostasis in the small intestine (Chen et al., Nature. 2021 May;593(7857):147-151;). We are now using in vivo animal models and in vitro biochemical approaches to interrogate CAR/Nr1i3-dependent gene regulatory mechanisms in CD4 T cell subsets. More broadly, we are also building new tools and workflows to systematically identify other NRs (and their endogenous ligands) that regulate immune responses, both in the gastrointestinal (GI) tract and diverse tumor microenvironments.
Endogenous functions of the MDR1 transporter in immune cells.

Multidrug-resistance 1 (MDR1) is an ATP-dependent efflux pump recognized for removing chemotherapeutic drugs from cancer cells. At the same time, both the strict evolutionary conservation of MDR1 orthologs in prokaryotes (e.g., bacteria) and its broad expression in a variety of healthy mammalian cell types and tissues suggest that MDR1 has broader and more fundamental functions in normal physiology. In the immune system, we have shown that MDR1 suppresses bile acid (BA)-induced oxidative stress in small intestinal CD4 T cells. We have further demonstrated that MDR1 is also highly and constitutively expressed in CD8 cytotoxic T lymphocytes (CTL), where MDR1 transport function is required to sustain CTL responses to both viral and intracellular bacterial infections. In each of these cases, loss of inhibition of MDR1 transport function leads to severe oxidative stress and increased cell death (Chen et al., J Exp Med. April 2020, e20201434). We are using a combination of in vitro and in vivo approaches to define the endogenous substrates and functions of MDR1-medaited transport in CTL, which we hypothesize will inform new approaches to augment protective immune responses to vaccines and cancer immunotherapies.
Translational studies of human inflammatory bowel disease (IBD)
Being housed within the Walter and Carole Young Center for Digestive Health at Dartmouth Health (DH-CDH), our lab leverages both internal and external collaborations with leading gastroenterologists to analyze cellular and molecular underpinnings of mucosal immune dysfunction and inflammation in human IBD patients. These studies include bulk and single cell (sc)RNA-seq analysis of mucosal biopsies, as well as serum and fecal metabolomcis, from patients with Crohn’s disease (CD), ulcerative colitis (UC) and CD or UC patients with or without primary sclerosing cholangitis (PSC).